Dr. Han-Ching Wang

Dr. Han-Ching Wang
Position:Distinguished Professor
Group:Agriculture & Aquaculture technology group
Research Interests:Crustacean Immunology, Crustacean Virology, Systems Biology, Multi-omics Analysis
E-mail:wanghc@mail.ncku.edu.tw
Room:89806
Room Tel:+886-6-2757575#58219
Laboratory Tel:+886-6-2757575#58224#810

|
School |
Department |
Country |
Degree |
Period |
|
National Taiwan University |
Institute of Zoology |
TW |
Ph.D |
2002 ~ 2007 |
|
National Taiwan University |
Institute of Oceanography |
TW |
M.S. |
2000 ~ 2002 |
|
Chung Shan Medical University |
Department of Public Health |
TW |
B.S. |
1996 ~ 2000 |

|
Institute |
Position |
Period |
| National Central Library | Director-General | 2024. 02 ~ now |
| World Organisation for Animal Health WSD reference lab | Leading expert | 2022~ now |
| World Organisation for Animal Health AHPND reference lab | Leading expert | 2022~ now |
| Department of Biotechnology and Bioindustry Sciences, NCKU |
Distinguished Professor |
2020. 08 ~ now |
|
International Center for the Scientific Development of Shrimp Aquaculture, NCKU |
Director |
2020. 02 ~ 2024.01 |
|
National Cheng Kung University |
University library Curator |
2019. 02 ~ 2024.01 |
|
Department of Biotechnology and Bioindustry Sciences, NCKU |
Professor |
2016. 08 ~ 2020. 07 |
|
Institute of Biotechnology, NCKU |
Associate Professor |
2012. 08 ~ 2016. 08 |
|
Institute of Biotechnology, NCKU |
Assistant Professor |
2008. 08 ~ 2012. 08 |

Aquaculture development aligns with the Sustainable Development Goals (SDGs), including SDG 2, SDG 13, and SDG 14. Asia is the largest producer of shrimp aquaculture, accounting for over 70% of the world's total production. For decades, the biggest challenge faced by shrimp industry has been infectious diseases, particularly white spot disease (WSD) and acute hepatopancreatic necrosis disease (AHPND), both of which are listed by the World Organization for Animal Health (OIE). My team uses an integrated systems-biology and omics approach, which includes metabolomics and lipidomics, to study the complex biological phenomena of WSD and AHPND pathogenesis. Our efforts are not limited to scientific research, but also to the development of effective and eco-friendly disease control strategies. To achieve this, comprehensive scientific knowledge is essential, and we always strive to apply our findings to address the problems of shrimp diseases. The shrimp aquaculture industry has already benefited from some of our discoveries, which have been used in developing biosecurity management plans. We are continuously improving our systems, strategies, and actions for shrimp aquaculture, and we believe that, involving the global shrimp community in research and industrial collaboration is imperative for facilitating shrimp aquaculture to grow successfully and sustainably.
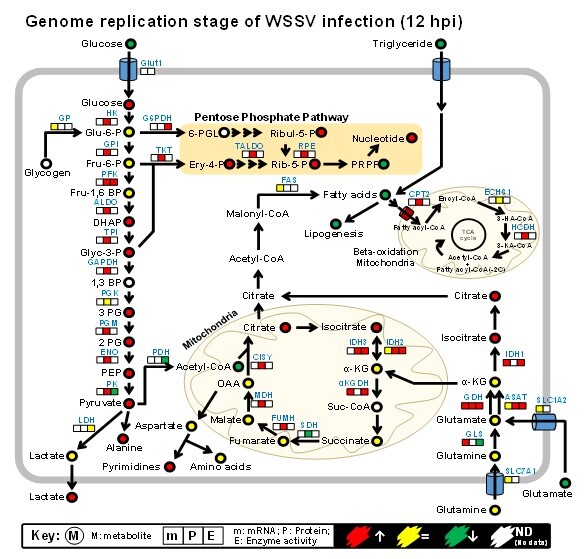 |
Discovery of a virus-induced Warburg effect in invertebrates Using an in vivo multi-omics animal model to (i) characterize the key host mechanisms for virus replication and (ii) elucidate the global interactions between shrimp and WSSV, my team and our collaborative research partners were the first to discover and report on the system-level metabolic changes caused by a viral infection in invertebrates. We documented the increased glucose consumption and plasma lactate concentrations that are induced by WSSV at the genome replication stage. This switch to aerobic glycolysis is also seen in human cancer cells, where it is known as the Warburg effect. Furthermore, the WSSV-induced Warburg effect is regulated by the RAS-PI3K-Akt-mTOR pathway, and it is essential for viral replication. Based on changes in the proteome and metabolome of WSSV-infected shrimp hemocytes, we showed that several other metabolic pathways, including nucleotide biosynthesis, glutaminolysis and amino acid biosynthesis, were induced at the WSSV genome replication stage (12 hpi). |
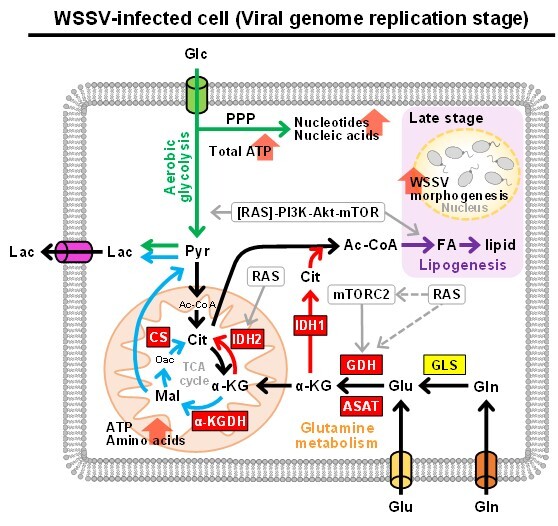 |
First report of virus-induced reductive glutamine metabolism in invertebrates To better understand WSSV’s impact on shrimp aerobic glycolysis and glutamine metabolism, we then developed the first in vivo stable isotope tracing metabolomics platform for this non-model animal using labeled isotopes ([U-13C]glucose, [U-13C]glutamine and [1-13C]glutamine). At the virus replication stage, both classic oxidative and distinct IDH1-mediated reductive glutamine metabolism were triggered, and both were found to be crucial for viral replication. Tracking of [U-13C] glutamine also showed that a considerable proportion of glutamine was converted into lactate via oxidative glutamine metabolism, suggesting that the accumulation of lactate, which is the hallmark of Warburg effect, may not only be due to the increased uptake of glucose, but may also come from glutamine. Further, since the significant IDH1-mediated reductive glutamine metabolism induced by WSSV might supply the essential bio-building blocks for lipogenesis at the late stage of WSSV replication, we are currently investigating the effects of suppressing the IDH1-mediated reductive glutamine metabolic pathway in the selection and breeding of WSSV-resistant shrimp. |
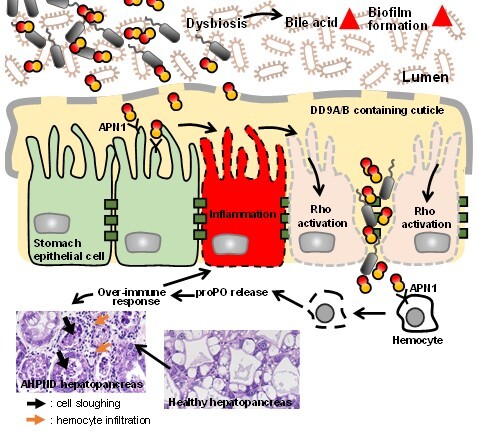 |
A comprehensive model of how AHPND-causing bacteria affect shrimp stomach and hepatopancreas Based on our scientific findings between 2017 and 2022 we proposed the comprehensive model of AHPND pathogenesis: 1. The shrimp stomach is composed of epithelial cells, a mucus layer (or cuticle) and a lumen with gut microbiota. AHPND-causing V. parahaemolyticus enter the shrimp stomach through an oral route. 2. The pathogen releases metabolites into the lumen, causing dysbiosis of gut microbiota. 3. As a first line of defense, the host secretes more bile acid into the stomach lumen. 4. Increased bile acid in the stomach induces protective biofilm formation by V. parahaemolyticus and the release of PirABVP toxins into the lumen. 5. Colonizing bacteria may disrupt the cuticle layer, stimulating an exaggerated immune response and Rho activation in shrimp stomach epithelial cells. 6. Activation of the Rho-signaling pathway disrupts stomach epithelial cell tight junctions. Formation of intercellular gaps enables PirABVP toxins and bacteria to pass into the hepatopancreas. In addition, PirABVP toxins interact with hemocytes by binding with APN1 and causing cell lysis. The proPO-mediated over-immune response is triggered, causing sloughing of hepatopancreatic cells. |

|
Name of Award |
Year of Award |
| College Student Research Creativity Award, MOST (Ministry of Science and Technology) | 2025 |
| The Asian Fisheries Society Gold Medal Award | 2025 |
| 2024 CH Biotech Innovation-Young Scholar Award | 2024 |
| Annual Outstanding Award for Supervising Doctoral Students | 2023 |
| Excellent Teacher Award, NCKU/span> | 2023 |
| International Outstanding Young Scholars (National Science and Technology Council Announcement for 2030 Cross-Generation Young Scholars Program) | 2022 |
| Advisor, 4th Prize in Engineering Taiwan International Science Fair | 2021 |
| Exemplary Teacher Award, 9th Venerable Master Hsing Yun Public Education Trust Fund | 2021 |
| College Student Research Creativity Award, MOST (Ministry of Science and Technology) | 2020 |
| Excellent USR Teacher Award, NCKU | 2020 |
| College Student Research Creativity Award, MOST (Ministry of Science and Technology) | 2020 |
| National Agricultural Science Award -- forward-looking innovation, Council of Agriculture, Executive Yuan, R. O. C | 2019 |
| Ta-You Wu Memorial Award Research Grant, MOST (Ministry of Science and Technology) | 2019 |
| Young Affiliate, TWAS (The World Academy of Science) | 2018 |
| Rising star" awards, 2018 Taiwan Outstanding Women in Science Awards | 2018 |
| Ta-You Wu Memorial Award, MOST (Ministry of Science and Technology) | 2016 |
| Research Award, Dr. K. T. Li Foundation | 2015 |
| The Shang-Fa Yang Young Scientist Award | 2014 |
| Agricultural Biotechnology Industrialization Development Program Project Summit Award and Model Case | 2013 |
| Excellent Teacher Award, NCKU | 2013 |
| Young Scholar Innovation Award, TCUS (Taiwan Comprehensive University System) | 2012 |
| Outstanding Young Investigator Award, NSC | 2012 |


.svg.png)
