Dr. Lynn L.H. Huang

Dr. Lynn L.H. Huang
Position:Distinguished Professor
Group:Biomedical
Research Interests:Regenerative medicine, biomaterials and tissue engineering
E-mail:lynn@mail.ncku.edu.tw
Room:89A03
Room Tel:+886-6-2757575#58227
Laboratory Tel:+886-6-2757575#58244#113

| School | Department | Country | Degree | Period |
| University of Southern California | Biochemistry, School of Medicine | U.S.A. | PhD | 1987.09 ~ 1993.05 |
| National Taiwan University (NTU) | Biochemistry & Nutrition, Inst. of Agricultural Chemistry | Taiwan | MS | 1983.09 ~ 1985.06 |
| National Taiwan University (NTU) | Department of Agricultural Chemistry | Taiwan | BS | 1979.09 ~ 1983.06 |

| Institute | Position | Period |
| Institute of Clinical Medicine, College of Medicine, NCKU | Distinguished Professor | 2017~present |
| Department of Biotechnology and Bioindustry Sciences, College of Bioscience and Biotechnology, NCKU | Distinguished Professor | 2017~present |
| Department of Biotechnology and Bioindustry Sciences, NCKU | Professor | 2015~2017 |
| Research Center of Excellence in Regenerative Medicine, NCKU | Director | 2010~2014 & 2016~present |
| Institute of Clinical Medicine, College of Medicine, NCKU | Professor | 2006~2017 |
| Department of Surgery, Stanford University School of Medicine, Palo Alto, California, U.S.A. | Visiting Professor | 2003-2004 |
| Institute of Biotechnology, NCKU | Professor | 1999~2017 |
| Institute of Biotechnology, National Cheng Kung University (NCKU) | Associate Professor | 1998~1999 |
| Graduate Institute of Oral Biology, NTU | Adjunct Associate Professor | 1997~2004 |
| Center for Biomedical Engineering, NTU | Research Associate Professor | 1994~1998 |
| Center for Biomedical Engineering, College of Medicine, NTU | Research Assistant Professor | 1993~1994 |

The BMT lab aims at the advancement of regenerative medicine, which includes research and product development in the fields of biomaterials, tissue engineering and cell therapy. With more than 92 international patents acquired in the last two decades, we combine knowledges and methods of biochemistry, cell biology, molecular biology, chemistry, material sciences and chemical engineering and dedicate to translate research results into industrial applications and solve the problems of clinical medicine. The core technologies in the BMT lab include production of non-reconstituted porous collagen matrix and concentrated collagen solution at high purity, expansion of stem cells while preserving their differentiation potentials, sterilization of biological materials, identification and quantification of collagen, creation of various wound dressings, novel biological adhesives, cell tissue gels, etc. to facilitate tissue regeneration. Through a stringent animal model, we also develop a first novel pharmaceutical regenerative medicine for treating critical limb ischemia effectively and preventing from amputation.
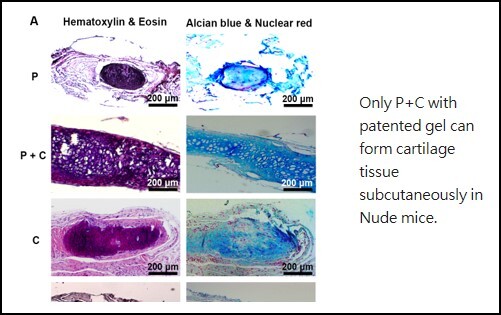 |
De Novo Cartilage Formation Regeneration of cartilage defects has been a key obstacle in clinical practice due to lacking proliferation capacity and dedifferentiation of mature chondrocytes. Since autologous chondrocyte transplantation has been used as a new direction for treating cartilage damage, this study provides a new approach to rejuvenate mature chondrocytes (C cells) to prolong their proliferation capacity and maintain chondrocyte characteristics through influencing by perichondrial progenitor cells (P cells). Our results demonstrate that the passage number of C cells can be prolonged from P6 to P9 and the cell number can be enlarged up to 128 times by co-cultured with P cells. Yet, the cells with chondrocyte characteristics increased cumulatively to 198 times by paracrine effect of P cells. The subcutaneous implantation study significantly demonstrated a synergetic effect of the interaction between P cells and C cells for chondrogenesis and formed an integral cartilage structure. These prominent effects should solve the problem of limited donor sites of cartilage and greatly improve huge needs of chondrocytes clinically. The method can be translated to clinical application directly in which perichondrium can be placed closely to a cartilage defect without extra manipulation of cells, or addition of any growth factors or induction medium. |
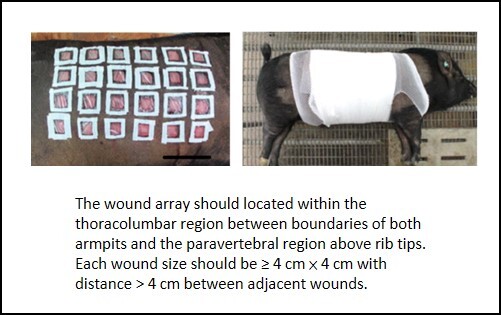 |
Golden Standard of a Wound Array in Pigs There is a tremendous need for an appropriate animal wound model to evaluate the effectiveness of various interventions such as wound dressings, cell therapies, and pharmaceutical agents. For translating research successfully, minipig was chosen owing to its similarity with human beings in aspects of body size, weight, and physiological status. In literatures, wounds of varying sizes were created at varying distances but fail to adequately distinguish the efficacy of various interventions. We attempted to resolve potential drawbacks by developing a systematic wound healing system. No significant variations in dorsal wound closure and contraction were observed within the thoracolumbar region between boundaries of both armpits and the paravertebral region above rib tips. This study has set some golden standards of optimal wound size (≥4 cm × 4 cm) and the minimum distance (˃4 cm) between adjacent wounds to effectively differentiate various interventions. Complied with the 3R principles of animal protocols, this study has established an optimized wound-array model for providing a high-throughput platform not only for precise screening effectiveness of different intervention treatments, but also for examining histological changes and for elucidating underlying molecular mechanisms in the process of wound healing. |
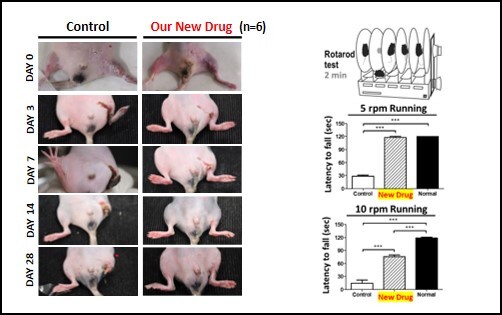 |
Novel Therapy for Critical Limb Ischemia We have developed a first novel pharmaceutical drug using the concept of regenerative medicine to fulfill an unmet clinical need for treating critical limb ischemia (CLI) effectively. Through a harsh assessment platform of a regeneration-deficient murine model with severe ischemic hindlimb, we have proved the success of Grace-001 for ischemic treatments. We have broken through the bottleneck of the existing technologies and have evaluated the efficacy of the drug stringently. Grace-001 can promote neovascularization, neonervation, tissue regeneration and restore blood flow as well as tissue functions. This new drug Grace-001 can effectively retain patients' non-necrotic tissues and prevent CLI limbs from amputation. This new drug not only saves patients' limbs and lives but also rescues economics and heavy burden on national health care. As it comprises of FDA-approved ingredients, its approval can be achieved under the 505(b)(2) regulatory pathway and applied to human through a Fast-tract Act. Grace-001 can greatly reduce costs, risk and time to the markets, making it highly advantageous over its competitive therapies such as stem cell therapy and gene therapy. There are more than 8.9 million CLI patients at an annual growth rate of 4.6% in 8 advanced countries with a market more than USD 3.1 billion. |

| Name of Award | Year of Award |
| Exceptional Research Award of the Ministry of Science and Technology, Taiwan | 2017 |
| Research Awards of the National Science Council/ the Ministry of Science and Technology (MOST), Taiwan | 1993~2018 |
| Special Outstanding Talent Award of the Ministry of Science and Technology through NCKU, Taiwan | 2012~2017 |
| Silver Medal Award at the Innovation and Entrepreneurship of Medical Biotechnology, Ministry of Education | 2015 |
| The 11th National Innovation Award, Academic Research Category: “Application and therapy of porcine embryonic stem cells on human disease models” | 2014 |
| The 11th National Innovation Award, Academic Research Category: “A novel high efficient extraction of collagen” | 2014 |
| Lifetime Achievement Academic Award for Outstanding International Achievement at the 10th International Inventor Prize | 2014 |
| Service Award of the Ministry of Education for the Pilot Educational Programs in Translational Medicine and Agriculture | 2013 |
| Pride of the Nation Academic Award for Outstanding International Achievement at the 8th and 10th International Inventor Prize | 2012 and 2014 |
| Outstanding Faculty Award of Industry-University Cooperation, NCKU | 2012 |
| Gold Medal Archimedes at the 15th Moscow International Salon of Inventions and Innovation Technologies | 2012 |
| Gold Medal at the 5th International Warsaw Invention Show “IWIS” | 2011 |
| The 7th National Innovation Award, Academic Research Category: “Platform technology of porous collagen matrix” | 2010 |
| Gold Medal of the “LiGuoDing Science Technology and Humanities” | 2010 |
| Fellow, Biomaterials Science and Engineering (FBSE) since | 2008 |


.svg.png)
