林士鳴 副教授

林士鳴 副教授
學群:農業及海洋生物科技產業學群
研究專長:結構生物學、酵素動力學、生物物理化學
E-mail:smlin@mail.ncku.edu.tw
研究室:89703
研究室Tel:+886-6-2757575#58210
實驗室Tel:+886-6-2757575#58214#714

| 學校 | 系所 | 國家 | 學位 | 起訖年月 |
| 國立清華大學 | 生物資訊與結構生物研究所 | 中華民國 | 博士 |

| 服務機關 | 職稱 | 起訖年月 |
| 國立成功大學 | 副教授 | 2024.02~至今 |
| 國立成功大學 | 助理教授 | 2016.02~2024.01 |

植物為了因應各種環境刺激的變化,會應用多種植物荷爾蒙和植化素來傳遞訊號並產生合適的反應。為此,這些植物荷爾蒙與植化素需要被精準的運輸與傳遞,進而使植物演化出一系列跨膜運輸蛋白,能夠精準辨識分子結構多元的植物荷爾蒙,並且以高度專一性與高靈敏度的方式進行跨膜運輸。我們團隊正嘗試解析這些運輸蛋白的生化特性與分子結構,藉此釐清這些序列相似的運輸蛋白,是如何辨識並運輸其對應的小分子受質,進而事實地調控植株整體的荷爾蒙分布。期望未來能應用於開發植物生長調節相關策略,以擴增農作產量並且減少化學肥料之使用,邁向永續農業之願景。
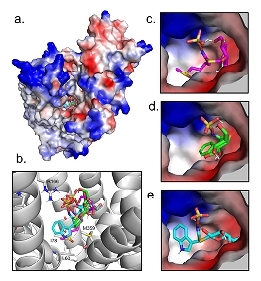 |
Glucosinolates (GLSs) are secondary metabolites that play a crucial role in plant defense against herbivores. In Arabidopsis thaliana, GLSs are transported via a proton gradient-driven process by Glucosinolate Transporter 1 (AtGTR1), which also transports phytohormones such as jasmonic acid-isoleucine (JA-Ile) and gibberellin (GA). However, little is known about the mechanisms underlying the broad substrate specificity of AtGTR1. To investigate the substrate preferences of AtGTR1, a yeast uptake assay was conducted, which showed that the transport rate of GLSs is negatively correlated with their hydrophobicity. AtGTR1 also showed a higher affinity for GLSs with higher hydrophobicity, suggesting a hydrophobic substrate binding pocket. Competition assays revealed that JA, salicylic acid (SA), and indole-3-acetic acid (IAA) can compete with GLS for transport in yeast, suggesting a potential interaction between AtGTR1 and these phytohormones. Mutagenesis experiments confirmed that the conserved EXXEK motif and Arg166 are essential for the GLS transport function of AtGTR1. The purified AtGTR1 adopts a homodimeric conformation, which is possibly regulated by phosphorylation on Thr105. The phosphomimetic mutation, T105D, reduced protein expression and completely abrogated the GLS transport function of AtGTR1, indicating the essential role of phosphorylation on AtGTR1. These findings enhance our understanding of how the distribution of defense GLSs is regulated in plants and could be applied to improve crop quality in agriculture. |
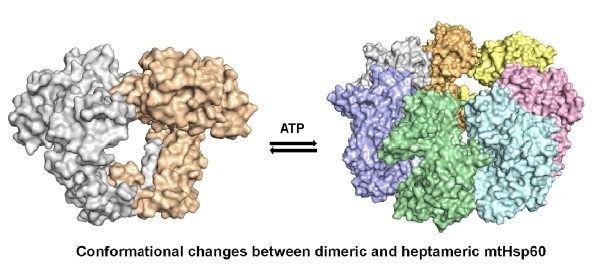 |
The proper folding of proteins in the mitochondria is crucial for maintaining cellular function, and Mitochondrial Hsp60 (mtHsp60) plays a significant role in this process. mtHsp60 is a tetradecamer, composed of two heptameric rings, but it tends to dissociate in vitro. However, the exact structure of dissociated mtHsp60 and the mechanism behind its dissociation remain unclear. Our research focuses on Epinephelus coioides mtHsp60 (EcHsp60), which can form a dimeric structure with inactive ATPase activity. The crystal structure of this dimer shows symmetrical subunit interactions and a rearranged equatorial domain. The α4 helix of each subunit extends and interacts with its adjacent subunit, leading to the disruption of the ATP-binding pocket. Additionally, the apical domain of the dimeric complex contains a highly conserved RLK motif that contributes to stabilizing the dimeric complex. These findings provide new insights into the conformational transitions and functional regulation of mtHsp60. The dimeric structure of EcHsp60 provides novel insight on the dissociation mechanism of mtHsp60 and offers a potential explanation for its instability in vitro. This study provides a foundation for future investigations into the functional regulation of mtHsp60 and its role in protein folding in the mitochondria. |

| 項目 | 獲獎年 |
| 第22屆王民寧獎之國內醫藥研究所博士班優秀論文獎 | 2012 |


.svg.png)
