王育民 特聘教授

王育民 特聘教授
學群:生物醫學科技產業學群
研究專長:發炎機制、癌症機轉、基因轉錄、訊息傳遞
E-mail:yumingw@mail.ncku.edu.tw
研究室:89906
研究室Tel:+886-6-2757575#58226
實驗室Tel:+886-6-2757575#58234#910

| 學校 | 系所 | 國家 | 學位 | 起訖年月 |
| 國防醫學院 | 生命科學研究所 | 臺灣 | 博士 | 1995.08 ~ 1999.12 |
| 台灣海洋大學 | 生物科技研究所 | 臺灣 | 碩士 | 1993.07 ~ 1995.07 |
| 輔仁大學 | 生物系 | 臺灣 | 學士 | 1989.09 ~ 1993.06 |

| 服務機關 | 職稱 | 起訖年月 |
| 國立成功大學 生物科技與產業科學系 | 代理系主任 | 2023.02~2023.07 |
| 國立成功大學 生物科學與科技學院 | 院長 | 2022.08~迄今 |
| 國立成功大學 教務處 | 教務長 | 2019.02~2022.07 |
| 國立成功大學 | 特聘教授 | 2016.08~迄今 |
| 國立成功大學 教務處 | 副教務長 | 2015.02~2019.01 |
| 國立成功大學 教學發展中心 | 主任 | 2015.02~ |
| 教育部雲嘉南區域教學資源中心 | 主任 | 2015.02~2017.12 |
| 生物資訊與訊息傳遞研究所 | 所長 | 2013.08~2017.07 |
| 生物科技與產業科學系 | 教授 | 2016.08~迄今 |
| 生物資訊與訊息傳遞研究所 | 教授 | 2012.09~2017.07 |
| 國立成功大學藥理所 | 合聘教授 | 2012.09~迄今 |
| 國立成功大學基礎醫學研究所 | 合聘教授 | 2012.09~迄今 |
| 生物資訊與訊息傳遞研究所 | 副教授 | 2009.09~2012.08 |
| 生物資訊研究所 | 合聘助理教授 | 2007.09~2010.08 |
| 訊息傳遞研究所 | 助理教授 | 2006.09~2009.08 |
| 德州大學安德森癌症研究中心 | 訪問學者 | 2018.06~2018.08 |
| 京都大學藥理學科 | 訪問學者 | 2012.07~2012.09 |
| 加州大學爾灣分校生物化學系 | 訪問學者 | 2011.07~2011.09 |
| 加州大學爾灣分校生物化學系 | 博士後研究員 | 2005.03~2006.08 |
| 成功大學藥理所 | 研究助理教授 | 2004.11~2005.02 |
| 成功大學藥理所 | 博士後研究員 | 2003.02~2004.10 |
| 中央研究院分子生物所 | 博士後研究員 | 2000.01~2003.01 |

發炎相關疾病(包括癌症)成因複雜且不易控制,如何有效控制發炎反應,以減緩甚至解決發炎性疾病,一直是發炎研究學者的努力目標。我們致力於解決「不正常發炎反應」所導致的各類問題,本實驗室長期針對發炎參與各類疾病發展進行分子機制探究與解析,找到合適發炎因子標的,據此開發抗發炎藥物或找尋較佳治療策略,以用於多種發炎相關疾病的治療。目前透過尋找及開發抑制「發炎關鍵蛋白」建構完善之研發平台,結合探究細胞間或蛋白質間相互作用所需的分子生物、細胞生物及生物化學基礎研究,用以進行致病機制探究、藥物開發、動物實驗驗證與醫用原料等一系列之技術與產品開發,為疾病治療提供解決方案和解釋藥物作用的原因。
.png) |
Due to the heterogeneity and high frequency of genome mutations in cancer cells, targeting vital protumour factors found in stromal cells in the tumour microenvironment may represent an ideal strategy in cancer therapy. However, the regulation and mechanisms of potential targetable therapeutic candidates need to be investigated. An in vivo study demonstrated that loss of pentraxin 3 (PTX3) in stromal cells significantly decreased the metastasis and growth of cancer cells. Clinically, our results indicate that stromal PTX3 expression correlates with adverse prognostic features and is associated with worse survival outcomes in triple-negative breast cancer (TNBC). We also found that transforming growth factor beta 1 induces PTX3 expression by activating the transcription factor CCAAT/enhancer binding protein delta in stromal fibroblasts. Following PTX3 stimulation, CD44, a PTX3 receptor, activates the downstream ERK1/2, AKT and NF-κB pathways to specifically contribute to the metastasis/invasion and stemness of TNBC MDA-MB-231 cells. Two types of PTX3 inhibitors were developed to disrupt the PTX3/CD44 interaction and they showed a significant effect on attenuating growth and restricting the metastasis/invasion of MDA-MB-231 cells, suggesting that targeting the PTX3/CD44 interaction could be a new strategy for future TNBC therapies. (Clin Transl Med. 2022; 12(1): e724.) |
.png) |
Fibrosing interstitial lung diseases (fILD) are potentially fatal with limited therapeutic options and no effective strategies to reverse fibrogenesis. Myofibroblasts are chief effector cells in fibrosis that excessively deposit collagen in the pulmonary interstitium and lead to progressive impairment of gaseous exchange. Plasma and lung specimens from patients with fILD were applied for detecting pentraxin 3 abundance by ELISA and Immunohistochemistry. Masson's trichrome and Sirius red stains and hydroxyproline assay were performed for assessing collagen accumulation in the lungs of bleomycin-exposed conditional Ptx3-deficient and PTX3-neutralizing antibody (αPTX3i)-treated mice. Downstream effectors including signaling pathways and fibrotic genes were examined for assessing CD44-involved PTX3-induced fibrosis in HFL1 and primary mouse fibroblasts. PTX3 was upregulated in the lungs and plasma of bleomycin-exposed mice and correlated with disease severity and adverse outcomes in fILD patients. Decreased collagen accumulation, attenuation of alveolar fibrosis and fibrotic markers, and improved lung function were observed in bleomycin-exposed conditional Ptx3-deficient mice. PTX3 activates lung fibroblasts to differentiate towards migrative and highly collagen-expressing myofibroblasts. Lung fibroblasts with CD44 inactivation attenuated the PI3K-AKT1, NF-κB, and JNK signaling pathways and fibrotic markers. αPTX3i mimic-based therapeutic studies demonstrated abrogation of the migrative fibroblast phenotype and myofibroblast activation in vitro. Notably, αPTX3i inhibited lung fibrosis, reduced collagen deposition, increased mouse survival, and improved lung function in bleomycin-induced pulmonary fibrosis. The present study reveals new insights into the involvement of the PTX3/CD44 axis in fibrosis and suggests PTX3 as a promising therapeutic target in fILD patients. (Clin Transl Med. 2022; 12(11): e1099.) |
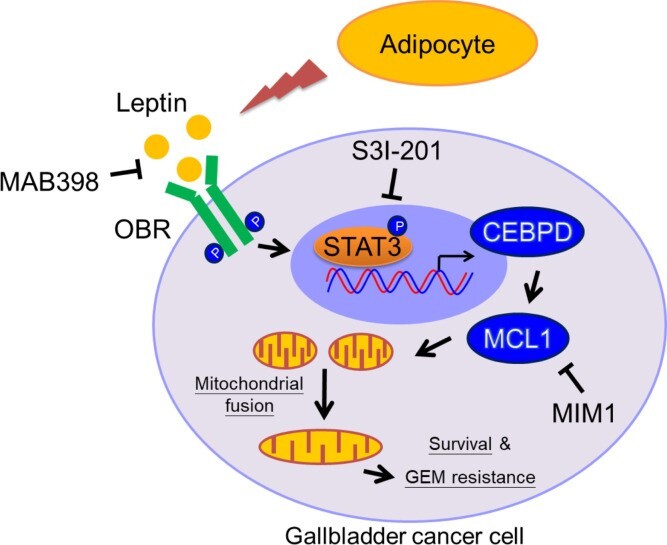 |
Obesity is a risk factor for gallbladder cancer (GBC) development, and it correlates with shorter overall survival. Leptin, derived from adipocytes, has been suggested to contribute to the growth of cancer cells; however, the detailed mechanism of leptin in GBC drug resistance remains uninvestigated. In this study, our finding that patients with GBC with a higher BMI were associated with increased GBC risks, including shortened survival, is clinically relevant. Moreover, obese NOD/SCID mice exhibited a higher circulating concentration of leptin, which is associated with GBC growth and attenuated gemcitabine efficacy. We further revealed that leptin can inhibit gemcitabine-induced GBC cell death through myeloid cell leukemia 1 (MCL1) activation. The transcription factor C/EBP δ (CEBPD) is responsive to activated STAT3 (pSTAT3) and contributes to MCL1 transcriptional activation upon leptin treatment. In addition, MCL1 mediates leptin-induced mitochondrial fusion and is associated with GBC cell survival. The findings in this study suggest the involvement of the pSTAT3/CEBPD/MCL1 axis in leptin-induced mitochondrial fusion and survival and provide a potentially new therapeutic target to improve the efficacy of gemcitabine in patients with GBC. (JCI insight, 2021; 6(15): e135438.) |

| 項目 | 獲獎年 |
| 113學年度李國鼎科技與人文講座-李國鼎榮譽學者 | 2024 |
| 李國鼎科技與人文講座-金質獎 | 2023 |
| 國立成功大學110年度產學特優奬 | 2022 |
| 109年度科技部傑出研究獎 | 2021 |
| 第十七屆國家創新獎續獎 | 2020 |
| 第十六屆國家創新獎續獎 | 2019 |
| 第十五屆國家創新獎(學研新創類) | 2018 |
| 科技部創新創業激勵計畫(FITI)–首獎 | 2018 |
| 台灣肝病防治基金會研究獎 | 2016 |
| 第十二屆國家新創獎 | 2015 |
| NHRI獎助三次里程碑獎 | 2014 |
| 第十一屆國家新創獎 | 2014 |
| Spandidos publications傑出成就獎 | 2014 |
| 台灣藥理學會李鎮源教授傑出研究獎 | 2013 |
| 國立成功大學教學優良教師 | 2013 |
| 科技部吳大猷獎 | 2010 |
| 台灣藥理學會年輕學者獎 | 2010 |
| 國立成功大學教學優良教師 | 2010 |
| 成杏基金會年度優秀論文獎 | 2007 |


.svg.png)
