羅玉枝 教授

羅玉枝 教授
學群:生物醫學科技產業學群
研究專長:生物化學、結構生物學、生物物理
E-mail:gracelo@ncku.edu.tw
研究室:89A04
研究室Tel:+886-6-2757575#58228
實驗室Tel:+886-6-2757575#58244#114

| 學校 | 系所 | 國家 | 學位 | 起訖年月 |
| 國防醫學大學 | 生命科學研究所 | 中華民國 | 博士 | |
| 國立台灣海洋大學 | 生物技術研究所 | 中華民國 | 碩士 |

| 服務機關 | 職稱 | 起訖年月 |
| 國立成功大學 | 教授 | 2025.08~迄今 |
| 國立成功大學 | 副教授 | 2017.08~2025.07 |
| 國立成功大學 | 助理教授 | 2011.02~2017.07 |
| 康乃爾大學 | 博士後研究員 | 2005.01~2011.01 |
| 中央研究所 | 博士後研究員 | 2004.05~2005.01 |

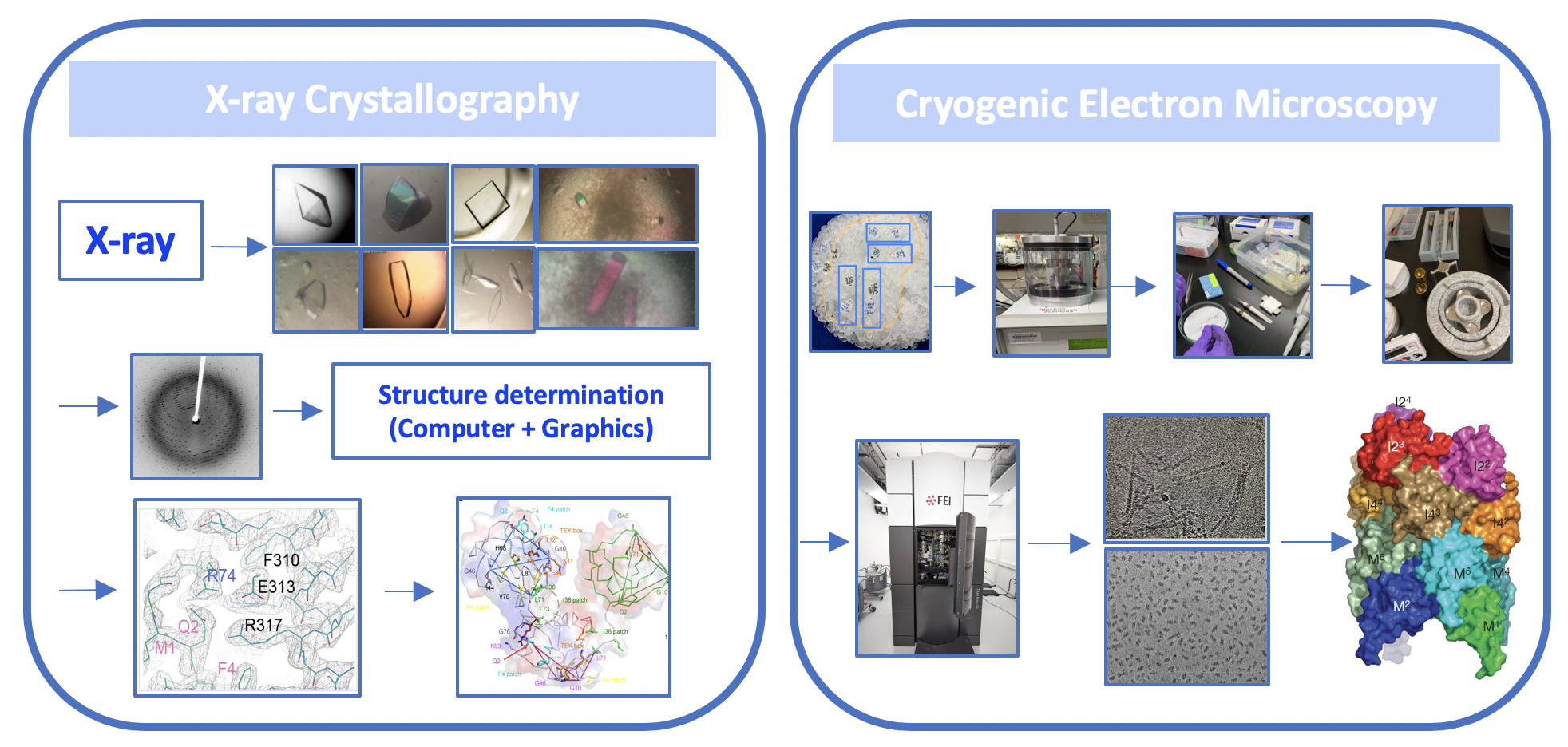
我們以結構生物學的研究為主。研究目標是以了解蛋白複合體在細胞生存訊息傳遞路徑、細胞表面死亡受體、與細胞內 Apoptosome 凋亡體,所介導的細胞生存與死亡訊息傳遞所參與的蛋白分子間的辨識與交互作用機制。同時,我們也對於人類病毒之非結構蛋白所參與的致病機制很有興趣。我們結合臨床病人的檢體進行實驗,以了解重要的病毒蛋白造成致病的分子機制。我們利用 X 光晶體學與冷凍電子顯微技術,結合了生化分析與細胞生物學的實驗方法,來達到這些研究目標。我們所取得的蛋白複合體的結構資訊與細胞死亡的訊息傳遞蛋白複合體辨識與組合的分子調控機制,有助於闡明這些參與訊息傳遞路徑中的相關蛋白質分子,因不正常調控所造成的先天免疫、細胞發炎、與細胞死亡等相關疾病或癌症,帶來新穎治療人類疾病的藥物設計和治療方法。
 |
Our research I. Explore Caspase8-cFLIP Complex Assembling in Tumor Cell Survival The findings provide insights into how tumor cells sustain survival and suggest strategies for designing targeted anti-cancer therapies that exploit these molecular features. II. Unravel Assembling of the FADD-procaspase-8-cFLIP Complex in Apoptosis This work advances the understanding of apoptosis and provides a structural basis for therapeutic interventions in diseases involving these pathways. III. Elucidate Enzymatically Active dimers of MALT1 by N+7 rule in BCL10-MALT1 Assembly The structural and functional insights pave the way for novel therapeutic strategies in immune and lymphoid malignancies. IV. A Potential Biomarker Discovery in Dengue Fever The findings establish λ FLC as a potential biomarker for disease diagnosis and severity assessment while shedding light on the viral immune evasion mechanisms. V. Met1-PolyUbs as a Multiple-molecule Binding Platform Our work provides a structural-functional glimpse of Met1-polyUb as a multiple-molecule binding platform to exert its intrinsic structural plasticity in mediating NF-κB signaling. We also identified ABIN1’s UBAN domain as a critical regulator that binds M1-polyubiquitin (Met1-polyUb), competing with other signaling molecules such as NEMO and RIP1. Structural analyses revealed that Met1-polyUb chains exhibit flexibility, facilitating the recruitment of multiple partner molecules to amplify signal transduction. These discoveries enhance our understanding of immune regulation and signal amplification, with implications for treating inflammation and autoimmune diseases.
|
期刊發表
KR Chen, HY Wang, YC Kuo, YC Lo, PL Kuo (2025) A novel SEPT12 mutation, T96I, is associated with sperm head and annulus defects. Front. in Cell Dev. Biol., 12:1498013.
CY Yang, YC Tseng, YF Tu, BJ Kuo, LC Hsu, CI Lien, YS Lin, YT Wang, YC Lu, TW Su, YC Lo*, and SC Lin* (2024). Reverse hierarchical DED assembly in the cFLIP-procaspase-8 and cFLIP-procaspase-8-FADD complexes. Nature Communications, 15:8974.
CY Yang, CI Lien, YC Tseng, YF Tu, A. W. Kulczyk, YC Lu, YT Wang, TW Su, LC Hsu*, YC Lo*, and Lin SC* (2024). Deciphering DED assembly mechanisms in FADD-procaspase-8-cFLIP complexes regulating apoptosis. Nature Communications, 15:3791.
BJ Kuo, SC Lin, YF Tu, PH Huang, YC Lo* (2024). Study of individual domains contributing to MALT1 dimerization in BCL10-independent and dependent assembly. Biochemical and Biophysical Research Communications, 717, 150029.
SH Wang, BJ Kuo, TC Ho, SW Wan, KL Yen, PH Huang, Oscar GC Perng, PL Chen, YW Chieng*, and YC Lo* (2023). Lambda-free light chain: A serum marker of dengue disease via NS3 protease-mediated antibody cleavage. Virulence, 14(1): 2279355.
JY Hong, SC Lin, BJ Kuo, YC Lo* (2021). Structural and biochemical basis for higher-order assembly between A20-binding inhibitor of NF-kB 1 (ABIN1) and M1-linked ubiquitins. J. Mol. Biol., 433; 167116
SM Lin, SC Lin, JY Hong, TW Su, BJ Kuo, WH Chang, YF Tu, YC Lo* (2017). Structural insights into linear tri-ubiquitin recognition by A20-bind-ing inhibitor of NF-κB (ABIN)-2. Structure, 2017; 25; 66-78.
TW Su, CY Yang, WP Kao, BJ Kuo, SM Lin, JY Lin, YC Lo*, SC Lin* (2017). Structural insights into DD-fold assembly and caspase-9 acti-vation by the Apaf-1 Apoptosome. Structure, 25; 407-420.
YC Lo and GC Perng* (2016). Novel concept on antiviral strategies to dengue. Current Opinion in Virology, 18; 97–108.
YC Lo*, SC Lin, CY Yang, JY Tung (2015). Tandem DEDs and CARDs suggest novel mechanisms of signaling complex assembly. Apoptosis, 20; 124-135.

| 項目 | 獲獎年 |
| 國立成功大學112學年度教學優良獎 | 2024 |
| American Heart Association Postdoctoral Fellow Award | 2009 |
| Cancer Research Institute Postdoctoral Fellow Award | 2005 |


.svg.png)
