羅玉枝 副教授

羅玉枝 副教授
學群:生物醫學科技產業學群
研究專長:生物化學、結構生物學、生物物理
E-mail:gracelo@ncku.edu.tw
研究室:89A04
研究室Tel:+886-6-2757575#58228
實驗室Tel:+886-6-2757575#58244#114

| 學校 | 系所 | 國家 | 學位 | 起訖年月 |
| 國防醫學大學 | 生命科學研究所 | 中華民國 | 博士 | |
| 國立台灣海洋大學 | 生物技術研究所 | 中華民國 | 碩士 |

| 服務機關 | 職稱 | 起訖年月 |
| 國立成功大學 | 副教授 | 2017.08~ |
| 國立成功大學 | 助理教授 | 2011.02~2017.07 |
| 康乃爾大學 | 博士後研究員 | 2005.01~2011.01 |
| 中央研究所 | 博士後研究員 | 2004.05~2005.01 |

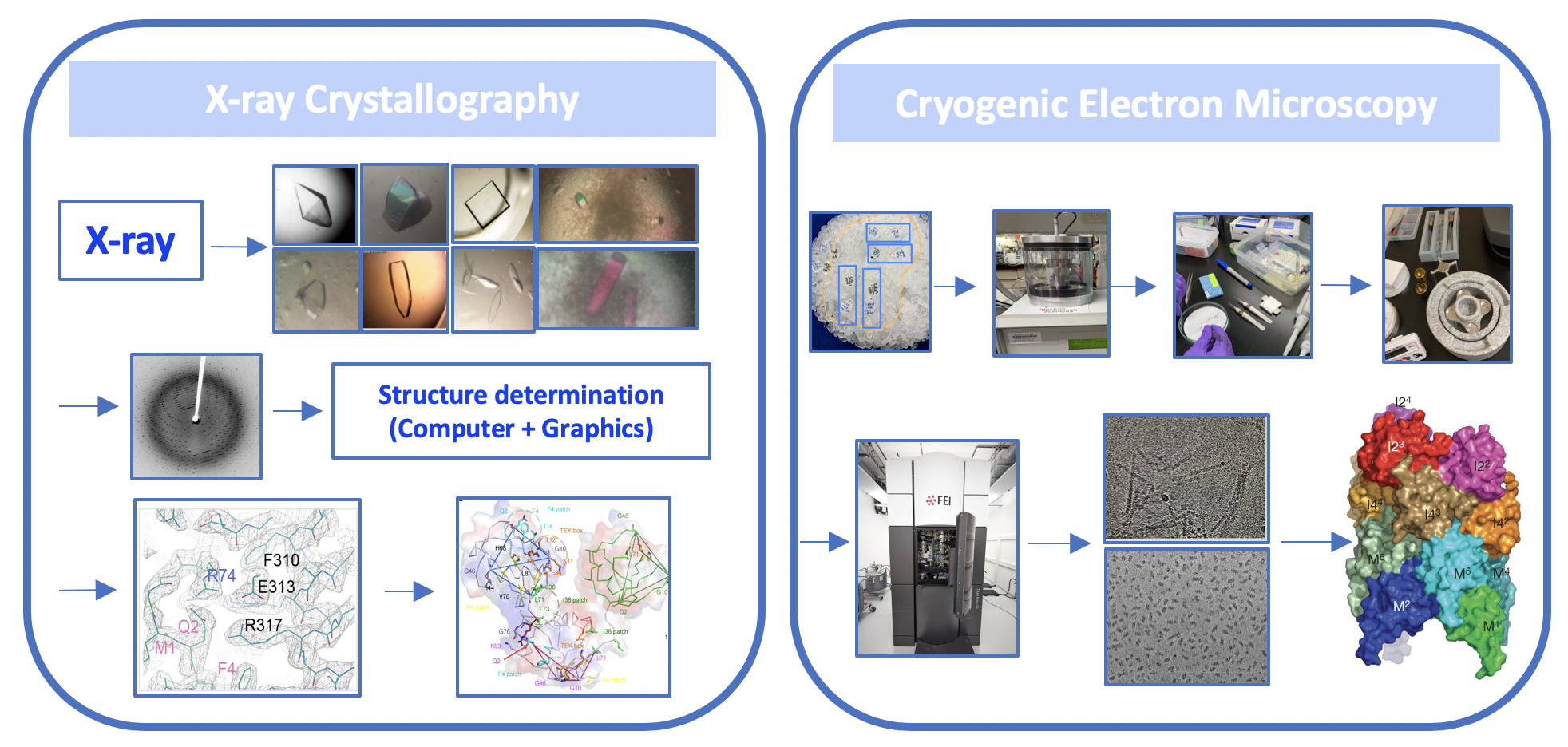
我們以結構生物學的研究為主。研究目標是以了解蛋白複合體在細胞生存訊息傳遞路徑、細胞表面死亡受體、與細胞內Apoptosome凋亡體,所介導的細胞生存與死亡訊息傳遞所參與的蛋白分子間的辨識與交互作用機制。同時,我們也對於人類病毒之非結構蛋白所參與的致病機制很有興趣。我們以in vitro也結合臨床病人的檢體進行in vivo實驗,以了解重要的病毒蛋白造成致病的分子機制。我們利用X光晶體學與冷凍電子顯微技術,結合了生化分析與細胞生物學的實驗方法,來達到這些研究目標。我們所取得的蛋白複合體的結構資訊與細胞死亡的訊息傳遞蛋白複合體辨識與組合的分子調控機制,有助於闡明這些參與訊息傳遞路徑中的相關蛋白質分子,因不正常調控所造成的先天免疫、細胞發炎、與細胞死亡等相關疾病或癌症,帶來新穎治療人類疾病的藥物設計和治療方法。
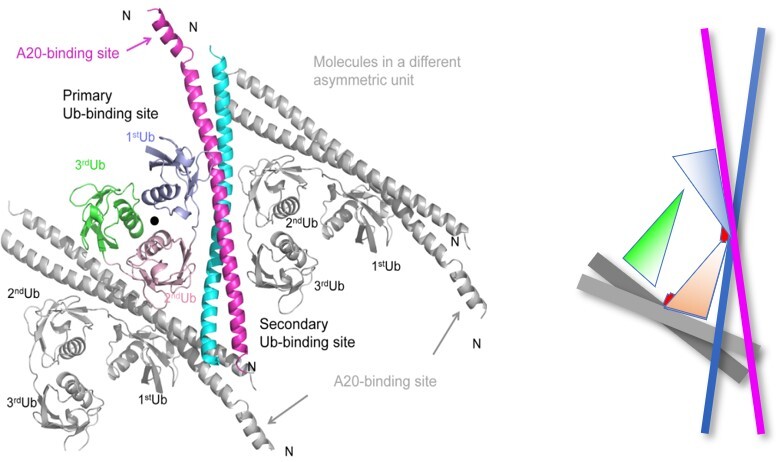 |
Our Research Polyubiquitination is crucial in controlling a number of cellular events. Polyubiquitins (polyUbs) are recognized by the functional diverse proteins with the ubiquitin-binding domain (UBD) to determine the signaling outcomes. During the past decade, many functional studies published in the high impact journals have pointed out that linear (M1-linked) polyUbs play a critical role in regulating the NF-κB signaling pathway. Excessive NF-κB activity has been linked to inflammatory disorders and autoimmune disease. We are interested in a M1-polyUbs binding protein family, the UBAN (ubiquitin-binding ABINs and NEMO) family, including NEMO, ABINs, and OPTN, which is crucial in mediating NF-kB signaling pathway. NEMO, a regulatory subunit of the IKK complex, acts as a positive regulator, whereas ABINs and OPTN play as a negative regulator in the NF-kB signaling. Among them, ABIN1 has been emerging as a preventer of inflammation and autoimmune to maintain immune homeostasis, which utilizes its UBAN domain to recognize linear (M1)-linked polyubiquitinated NF-κB activation mediators. PolyUb-mediated UBAN recruitment remains undetermined, since the recognition studies focused mostly on di-ubiquitin (diUb). The structural information of the detail molecular recognition mechanism for longer linear polyUbs is not well understood. How a polyUb promotes the interaction with ubiquitin-binding adaptors also remains largely unknown. Structural information for answering the questions is fundamentally important.
|

| 項目 | 獲獎年 |
| 國立成功大學112學年度教學優良獎 | 2024 |
| American Heart Association Postdoctoral Fellow Award | 2009 |
| Cancer Research Institute Postdoctoral Fellow Award | 2005 |
