王涵青 特聘教授

王涵青 特聘教授
學群:農業及海洋生物科技學群
研究專長:蝦類暨魚類微生物及免疫學
E-mail:wanghc@mail.ncku.edu.tw
研究室:89806
研究室Tel:+886-6-2757575#58219
實驗室Tel:+886-6-2757575#58224#810

| 學校 | 系所 | 國家 | 學位 | 起訖年月 |
| 國立台灣大學 | 動物學 | 台灣 | 博士 | 2002~2007 |
| 國立台灣大學 | 海洋研究所 | 台灣 | 碩士 | 2000~2002 |
| 中山醫學大學 | 公共衛生 | 台灣 | 學士 | 1996~2000 |

| 服務機關 | 職稱 | 起訖年月 |
| 國家圖書館 | 館長 | 2024.02~至今 |
| World Organisation for Animal Health WSD reference lab | Leading expert | 2022~至今 |
| World Organisation for Animal Health AHPND reference lab | Leading expert | 2022~至今 |
| 國立成功大學生物科技與產業科學系 | 特聘教授 | 2020.08~至今 |
| 國立成功大學前瞻蝦類養殖國際研發中心 | 主任 | 2020.02~2024.01 |
| 國立成功大學圖書館 | 館長 | 2019.02~2024.01 |
| 國立成功大學 | 教授 | 2016.08~2020.07 |
| 國立成功大學 | 副教授 | 2012.08~2016.08 |
| 國立成功大學 | 助理教授 | 2008.08~2012.08 |

水產養殖緊扣聯合國永續發展目標之SDG 2 、13和SDG,被視為當代重要趨勢產業。全球超過70%以上的養殖蝦類供應量來自於亞洲,可知亞洲是世界養殖蝦最重要生產地,然而此產業最大發展威脅是疾病問題,尤其是世界動物衛生組織表列疾病—蝦白點病(WSD)及急性肝胰腺壞死症(AHPND),已經造成全球養蝦產業難以估計經濟損失,但對其病原體與致病機轉解析仍非常有限。有鑑於此,我與我的團隊利用系統生物學以及多體學研究策略,研究蝦隻感染WSD和AHPND的致病機制及複雜相互作用關係。除了學術研究外,我們也致力於解決蝦類養殖產業所面對的疾病問題,利用全面性的科學知識來發展有效且環境友善的疾病控制策略,制定養蝦產業所需的生物安全性管理系統。我與我的團隊也持續推動與國際學產界合作,與世界連結而從生物特性到蝦類養殖環節等學術或實務上進行精進與提昇, 回應世界對於水產養殖之需求,也期待對世界永續發展有所貢獻。
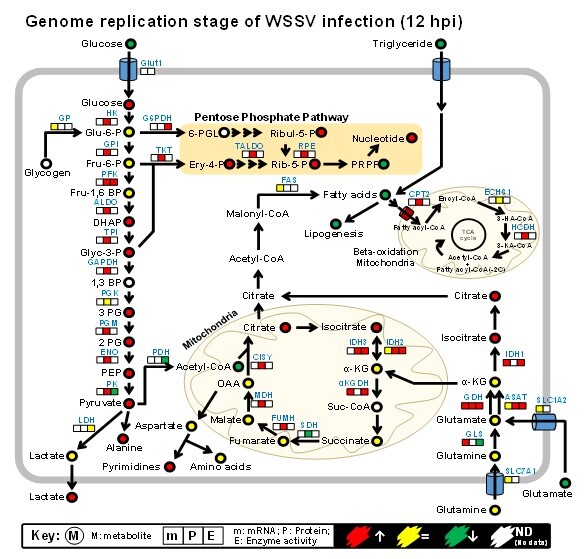 |
Discovery of a virus-induced Warburg effect in invertebrates Using an in vivo multi-omics animal model to (i) characterize the key host mechanisms for virus replication and (ii) elucidate the global interactions between shrimp and WSSV, my team and our collaborative research partners were the first to discover and report on the system-level metabolic changes caused by a viral infection in invertebrates. We documented the increased glucose consumption and plasma lactate concentrations that are induced by WSSV at the genome replication stage. This switch to aerobic glycolysis is also seen in human cancer cells, where it is known as the Warburg effect. Furthermore, the WSSV-induced Warburg effect is regulated by the RAS-PI3K-Akt-mTOR pathway, and it is essential for viral replication. Based on changes in the proteome and metabolome of WSSV-infected shrimp hemocytes, we showed that several other metabolic pathways, including nucleotide biosynthesis, glutaminolysis and amino acid biosynthesis, were induced at the WSSV genome replication stage (12 hpi). |
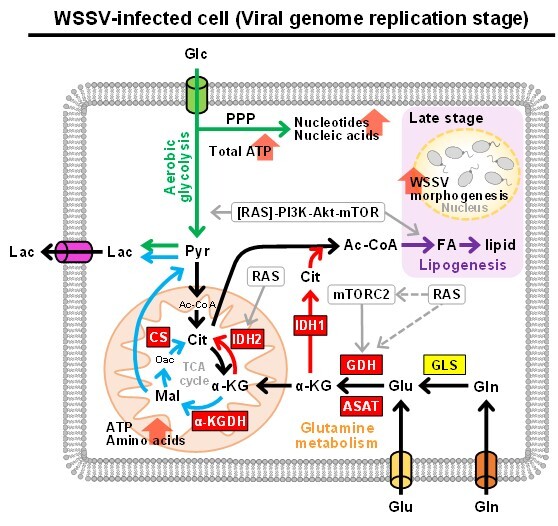 |
First report of virus-induced reductive glutamine metabolism in invertebrates To better understand WSSV’s impact on shrimp aerobic glycolysis and glutamine metabolism, we then developed the first in vivo stable isotope tracing metabolomics platform for this non-model animal using labeled isotopes ([U-13C]glucose, [U-13C]glutamine and [1-13C]glutamine). At the virus replication stage, both classic oxidative and distinct IDH1-mediated reductive glutamine metabolism were triggered, and both were found to be crucial for viral replication. Tracking of [U-13C] glutamine also showed that a considerable proportion of glutamine was converted into lactate via oxidative glutamine metabolism, suggesting that the accumulation of lactate, which is the hallmark of Warburg effect, may not only be due to the increased uptake of glucose, but may also come from glutamine. Further, since the significant IDH1-mediated reductive glutamine metabolism induced by WSSV might supply the essential bio-building blocks for lipogenesis at the late stage of WSSV replication, we are currently investigating the effects of suppressing the IDH1-mediated reductive glutamine metabolic pathway in the selection and breeding of WSSV-resistant shrimp. |
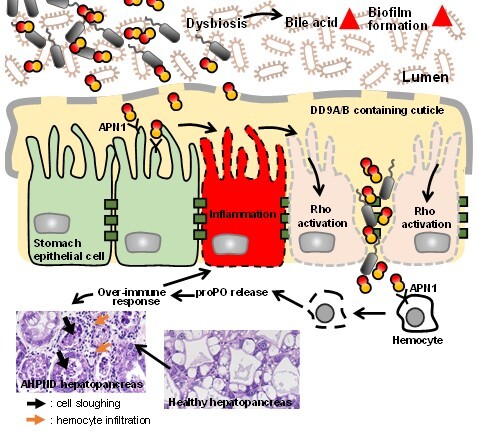 |
A comprehensive model of how AHPND-causing bacteria affect shrimp stomach and hepatopancreas Based on our scientific findings between 2017 and 2022 we proposed the comprehensive model of AHPND pathogenesis: 1. The shrimp stomach is composed of epithelial cells, a mucus layer (or cuticle) and a lumen with gut microbiota. AHPND-causing V. parahaemolyticus enter the shrimp stomach through an oral route. 2. The pathogen releases metabolites into the lumen, causing dysbiosis of gut microbiota. 3. As a first line of defense, the host secretes more bile acid into the stomach lumen. 4. Increased bile acid in the stomach induces protective biofilm formation by V. parahaemolyticus and the release of PirABVP toxins into the lumen. 5. Colonizing bacteria may disrupt the cuticle layer, stimulating an exaggerated immune response and Rho activation in shrimp stomach epithelial cells. 6. Activation of the Rho-signaling pathway disrupts stomach epithelial cell tight junctions. Formation of intercellular gaps enables PirABVP toxins and bacteria to pass into the hepatopancreas. In addition, PirABVP toxins interact with hemocytes by binding with APN1 and causing cell lysis. The proPO-mediated over-immune response is triggered, causing sloughing of hepatopancreatic cells. |

| 項目 | 獲獎年 |
| 113年指導學生-王懷勤 國科會大專學生「研究創作獎」 | 2025 |
| 亞洲水產學會-金牌獎 | 2025 |
| 正瀚生技創新-青年學者獎 | 2024 |
| 112年度 指導博士生-博士生傑出獎紀念 | 2023 |
| 111學年度 特優教師獎-教學傑出教師 | 2023 |
| 國科會2030跨世代年輕學者方案-國際年輕傑出學者 | 2022 |
| 臺灣國際科學展覽會工程學科四等獎-指導彰化高中粘恩睿同學 | 2021 |
| 第九屆星雲教育獎「典範教師獎」 | 2021 |
| 研究創作獎-蘇廣晨-科技部108年度大專學生參與專題計畫 | 2020 |
| 108學年度大學創新與大學社會責任之教學優良獎 | 2020 |
| 國科會108年度大專學生參與專題計畫研究創作獎; 指導教授王涵青 | 2020 |
| 國家農業科學獎之前瞻創新類千里馬獎 | 2019 |
| 國科會 吳大猷先生紀念獎研究計畫 | 2019 |
| 世界科學院年輕學者 | 2018 |
| 第11屆「台灣傑出女科學家獎」新秀獎 | 2018 |
| 科技部105年「吳大猷先生紀念獎」 | 2016 |
| 104學年度李國鼎科技與人文講座研究獎 | 2015 |
| 楊祥發院士傑出農業科學年輕學者獎 | 2014 |
| 農業生物技術產業化發展方案計畫登峰獎暨典範案例 | 2013 |
| 國立成功大學101學年度教學傑出教師 | 2013 |
| 台灣綜合大學系統年輕學者創新研究佳作 | 2012 |
| 國科會101年度優秀年輕者四年期研究計畫獎勵 | 2012 |


.svg.png)
